Ammonium nitrate, which recently caused an explosion in Delhi, has also been used in several major bomb blasts in Mumbai in the past when mixed with other chemicals.
About Ammonium Nitrate
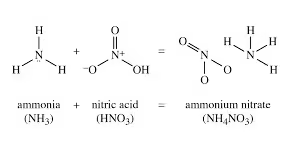
- Ammonium nitrate (NH₄NO₃) is a white crystalline solid produced on a large industrial scale.
- It is a chemical compound formed from ammonia and nitric acid.
- Widely used in agriculture as a nitrogen-rich fertilizer, it is also an oxidizing agent in industrial explosives.
- It melts at 170°C and dissolves easily in water. When heated in solution, it releases nitrous oxide (laughing gas).
- It is a key ingredient in commercial explosives, especially those used in mining operations.
Why Can Ammonium Nitrate Explode?
- In its pure form, ammonium nitrate is not explosive.
- It becomes explosive only when combined with fuels or other chemicals.
- To trigger an explosion, detonators or other initiators are required.
Legal Regulation in India
- As per the 2012 rules (updated in 2021), any mixture with over 45% ammonium nitrate is treated as an explosive.
- A District Magistrate can allow possession of up to 30 metric tonnes, but larger quantities need approval from PESO (Petroleum and Explosives Safety Organisation).
- PESO provides licenses for storage, transport, manufacturing, and use of ammonium nitrate.
This topic is available in detail on our main website.





